WHO ‘strongly recommends’ Pfizer's Covid-19 pill for at-risk patients: Report
The WHO experts said that US pharma giant Pfizer’s combination of nirmatrelvir and ritonavir was the “superior choice” of treatment for unvaccinated, elderly or immunocompromised people with Covid-19, according to AFP.
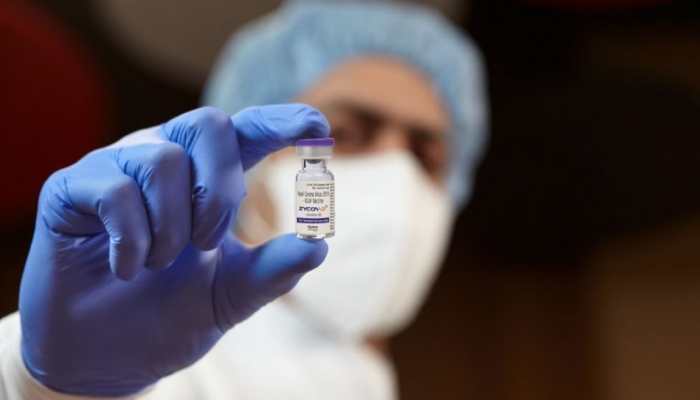
Apr 22, 2022, 08:16 AM ISTZydus Cadila's COVID-19 vaccine ZyCoV-D to be initially introduced in THESE seven states
Union Health Secretary Rajesh Bhushan directed these states to identify the districts with a high number of beneficiaries who have not taken even the first dose of the COVID-19 vaccine.
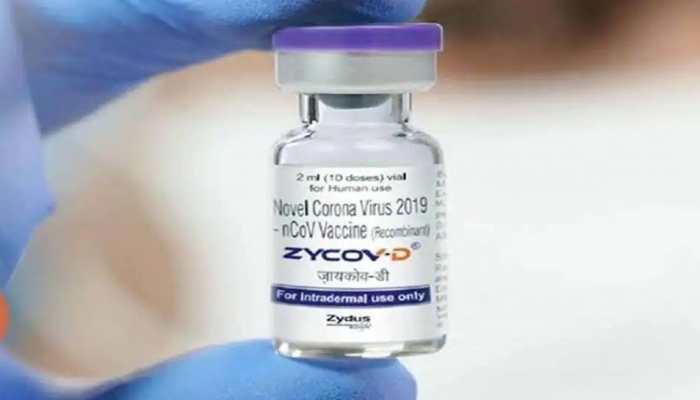
Dec 02, 2021, 16:06 PM ISTCentre places order for one crore doses of Zydus Cadila's COVID vaccine ZyCoV-D
The Union Health Ministry is learnt to have given the go ahead to initiate the preparatory work for the introduction of the indigenously developed ZyCoV-D.
Nov 07, 2021, 14:25 PM ISTIndia to procure over 30 crore COVID-19 vaccine doses per month by January: Sources
India will have a capacity of more than 30 crores by next year per month, which includes Covishield, Covaxin, ZyCoV-D and Biological E, said sources.
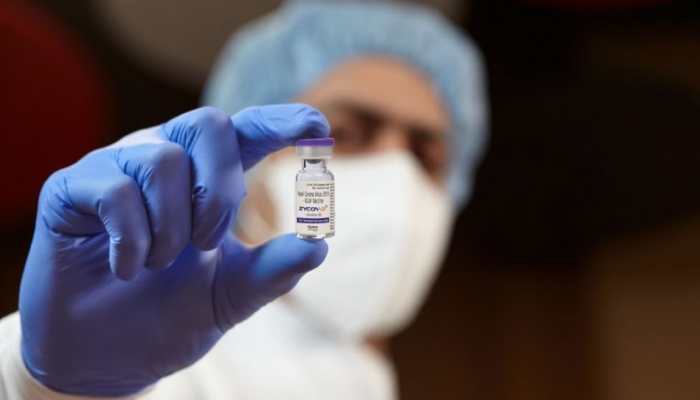
Oct 16, 2021, 15:33 PM ISTIndia first to develop DNA vaccine, can be administered to those above 12: PM Modi
Last month, the Drugs Controller General of India gave Emergency Use Authorisation (EUA) to Zydus Cadila's indigenously developed needle-free COVID-19 vaccine, ZyCoV-D, which is to be administered to beneficiaries in the age group of 12-18 years in the country.
Sep 26, 2021, 09:43 AM ISTIndia to roll out COVID-19 vaccine for children aged 12 and above from October: Report
From October, Zydus Cadila will produce 10 million doses a month of the world`s first DNA-based COVID-19 vaccine ZyCoV-D.
Sep 22, 2021, 19:57 PM ISTNTAGI to introduce Zydus Cadila's ZyCoV-D jab in vaccination drive, prioritise 12-18 age group with comorbidities
On ZyCoV-D vaccine, NTAGI Chairman said, “The aim is to develop a priority list with the focus being on adolescents aged 12-18 years with comorbidities.”
Aug 25, 2021, 16:53 PM ISTPriority COVID-19 vaccination for children above 12 with comorbidities: Govt panel chief
NTAGI chief said that a portion of Zydus Cadila’s Zycov-D vaccine supply will be set aside for comorbid adolescents. The announcement came days after the Drug Controller General of India (DCGI) granted emergency approval to the Zycov-D.
Aug 24, 2021, 07:35 AM ISTZydus Cadila hopes to begin supply of ZyCoV-D vaccine by mid to end September, says MD Sharvil Patel
Patel also said that the pricing of the ZyCoV-D dose will be announced in the next one or two weeks.
Aug 22, 2021, 07:25 AM ISTZydus Cadila's ZyCoV-D - the needle-free COVID-19 vaccine for adults and kids - all you need to know
Zydus Cadila in July had said that ZyCoV-D was effective against the new mutants of COVID-19, especially the Delta variant.
Aug 21, 2021, 10:22 AM ISTZydus Cadila's COVID vaccine ZyCoV-D, first jab for children in India, gets approval
Zydus Cadila Covid vaccine ZyCoV-D can be administered to people 12 years and above.
Aug 20, 2021, 20:57 PM ISTZydus Cadila likely to get emergency use approval for its COVID vaccine this week: Sources
Zydus Cadila had said last month that it has applied for emergency use authorisation (EUA) with the Indian Drug regulator for its three-dose COVID-19 vaccine ZyCoV-D, and plans to manufacture 10-12 crore doses annually.
Aug 09, 2021, 21:30 PM ISTZydus Cadila seeks emergency use approval for COVID vaccine for 12 years old and above
The company said that it has applied to the country`s drug regulator for emergency use approval of its COVID-19 vaccine and that it plans to manufacture up to 120 million doses of the shot annually.
Jul 01, 2021, 09:09 AM ISTWe will have an amazing New Year Today, After Vaccination approval?
The approval for a Covid vaccine can come soon, the country's drugs control authority has indicated.
Jan 01, 2021, 12:06 PM ISTCorona Vaccine : Pfizer Seeks India Approval For Covid Vaccine
Pfizer has sought approval from the country's drug regulator - the DCGI (Drugs Controller General of India) - for emergency use authorization of its coronavirus vaccine.
Dec 06, 2020, 14:12 PM ISTCoronavirus Update : How will people get Corona Vaccine?
Experts believe that a COVID vaccine "will be ready in the next few weeks", Prime Minister Narendra Modi said. Watch the full Video.
Dec 05, 2020, 08:42 AM IST