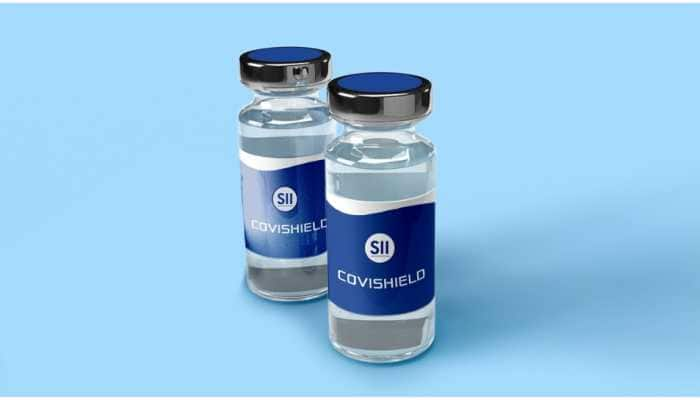
DCGI allows Serum Institute to conduct phase II+III trials of Oxford University vaccine COVISHIELD for COVID-19
According to the Indian Council of Medical Research, the COVID-19 tests around India crossed a two-crore mark on Monday.
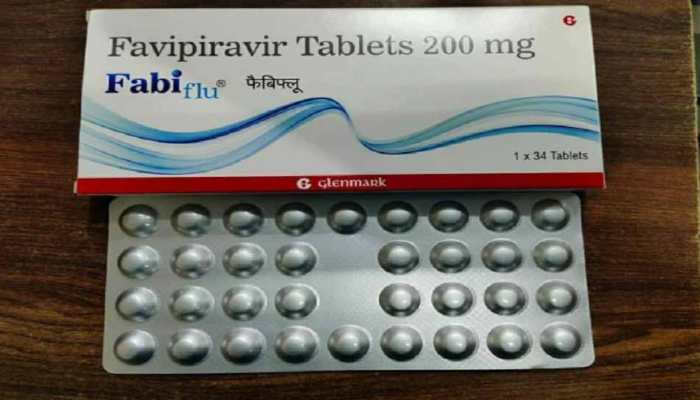
Aug 03, 2020, 17:53 PM ISTDCGI queries Glenmark Pharma on claims of FabiFlu use for COVID-19 patients with comorbidities
India's drug regulator DCGI has sought a clarification from Glenmark Pharmaceuticals over its alleged "false claims" about the use of anti-viral FabiFlu on COVID-19 patients with comorbidities.
Jul 19, 2020, 20:38 PM ISTDCGI gives nod for 'restricted emergency' use of Itolizumab injection on COVID-19 patients
Biocon has presented the Phase II clinical trial results generated in COVID-19 patients to DCGI.
Jul 11, 2020, 12:56 PM ISTDelhi High Court to resume hearing on petitions against Centre's ban on drugs
Delhi High Court is likely to resume today the hearing on petitions by over 30 pharmaceutical majors, including Pfizer, Glenmark, Procter and Gamble (P&G) and Cipla, challenging the government's decision to ban sale of some of their fixed dose combination medicines.
Mar 21, 2016, 07:32 AM ISTDelhi HC allows DCGI to process Reliance's package insert of cancer drug
Delhi High Court today told the Drug Controller General of India (DCGI) that it can process the application of Reliance Life Sciences for approving the package insert of its breast cancer drug, the launch of which has been put on hold by a single judge of the court.
Nov 19, 2015, 20:46 PM ISTGovt initiates steps to promote clinical research in India
The government has initiated a series of steps to fast-track approvals for clinical trials in India.
Nov 13, 2015, 12:11 PM IST