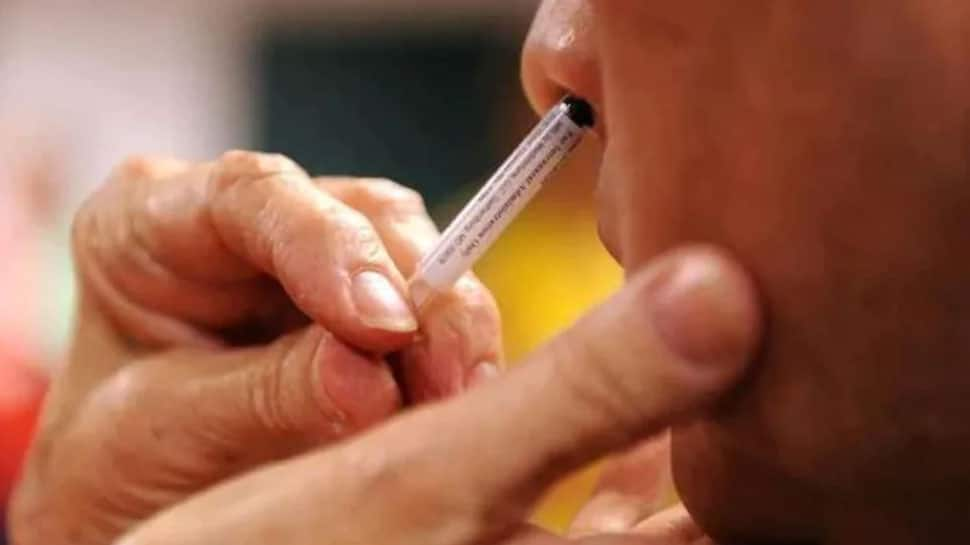
New Delhi: All India Institute of Medical Sciences (AIIMS) in Delhi will be starting Phase 2/3 trials of Hyderabad-based COVID-19 vaccine manufacturer Bharat Biotech`s nasal vaccine soon, according to informed sources.
Amid ongoing research in many countries to develop nasal spray to help prevent COVID -19, Bharat Biotech`s intranasal vaccine received regulatory approval for second Phase trials in August.
Sources said that the country`s premier hospital and research center will be starting trials within a couple of weeks and an application has been put to seek the mandatory permission of the AIIMS Ethics Committee.
Bharat Biotech`s nasal vaccine clinical trial`s principal investigator will be Dr Sanjay Rai. After getting the ethics committee`s nod, the second phase of the trials will be conducted on volunteers who will be administered the two doses of the vaccine with a gap of four weeks in between.
The adenoviral intranasal vaccine BBV154 is the first of its kind COVID-19 vaccine to undergo human trials in India. According to the ministry of science and technology, the Phase 1 trial in healthy volunteers of age groups ranging from 18-60 years was well tolerated.
Trials for the Phase 3 trial will commence after the completion of Phase-2 clinical trials. AIIMS Delhi has also done clinical trials of Covaxin in the age group of 2 to 18 years.